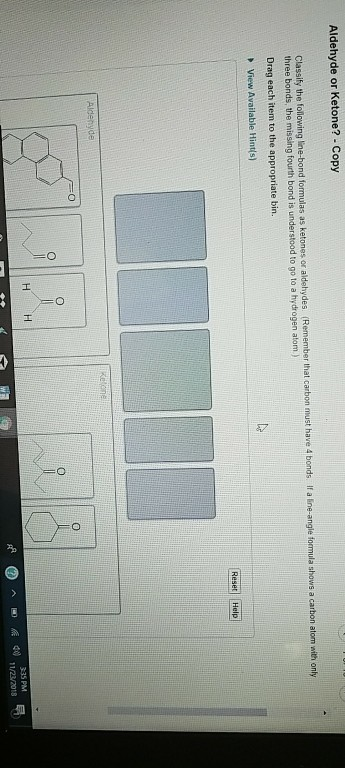
Drag each item to the appropriate bin. R and R can be any kind of carbon containing structure.
Solved Classity The Following Line Bond Formulas As Ketones Chegg Com
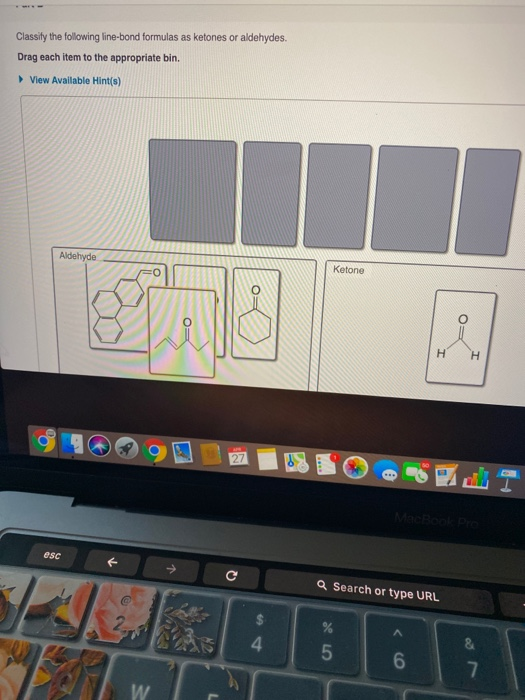
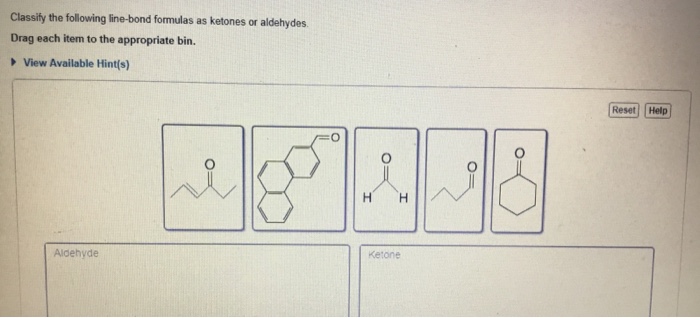
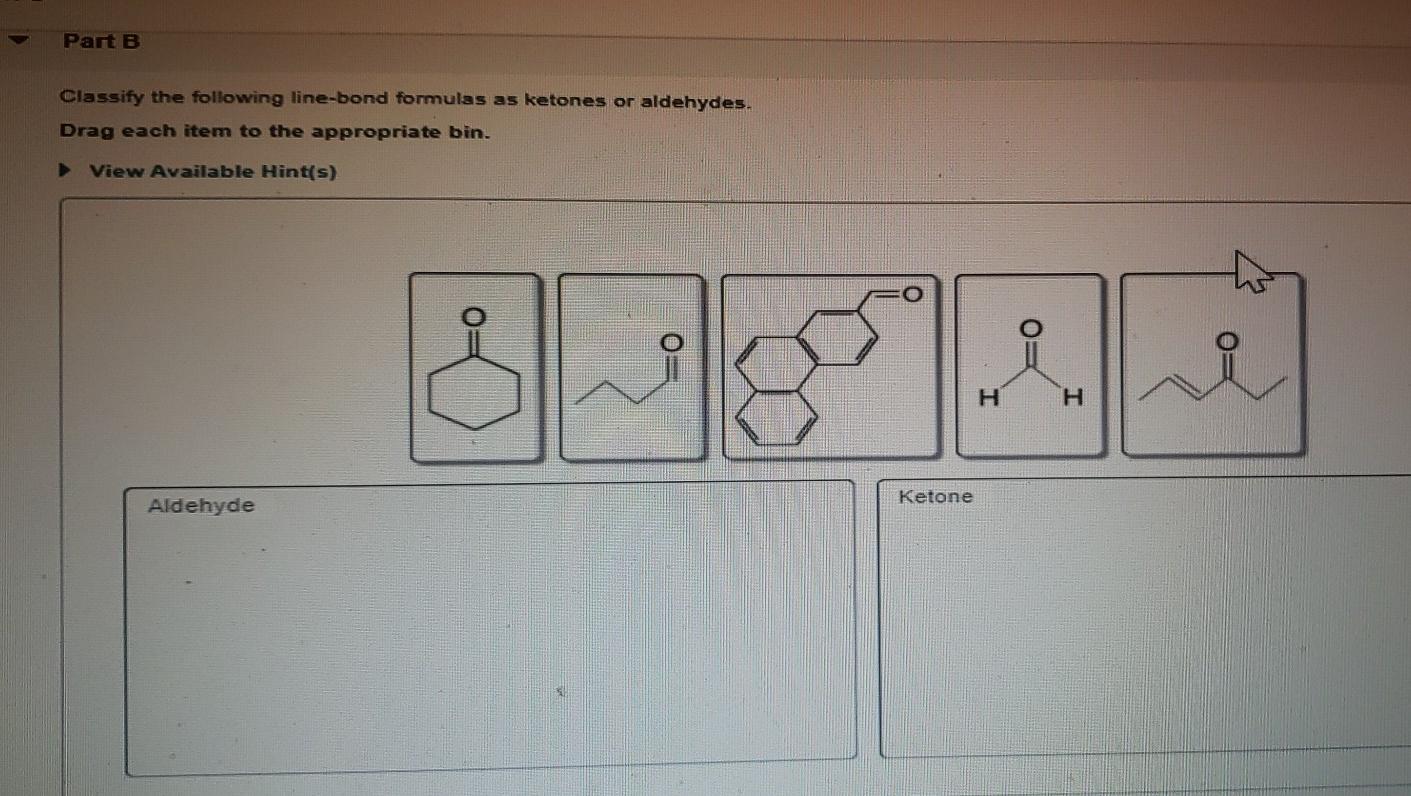
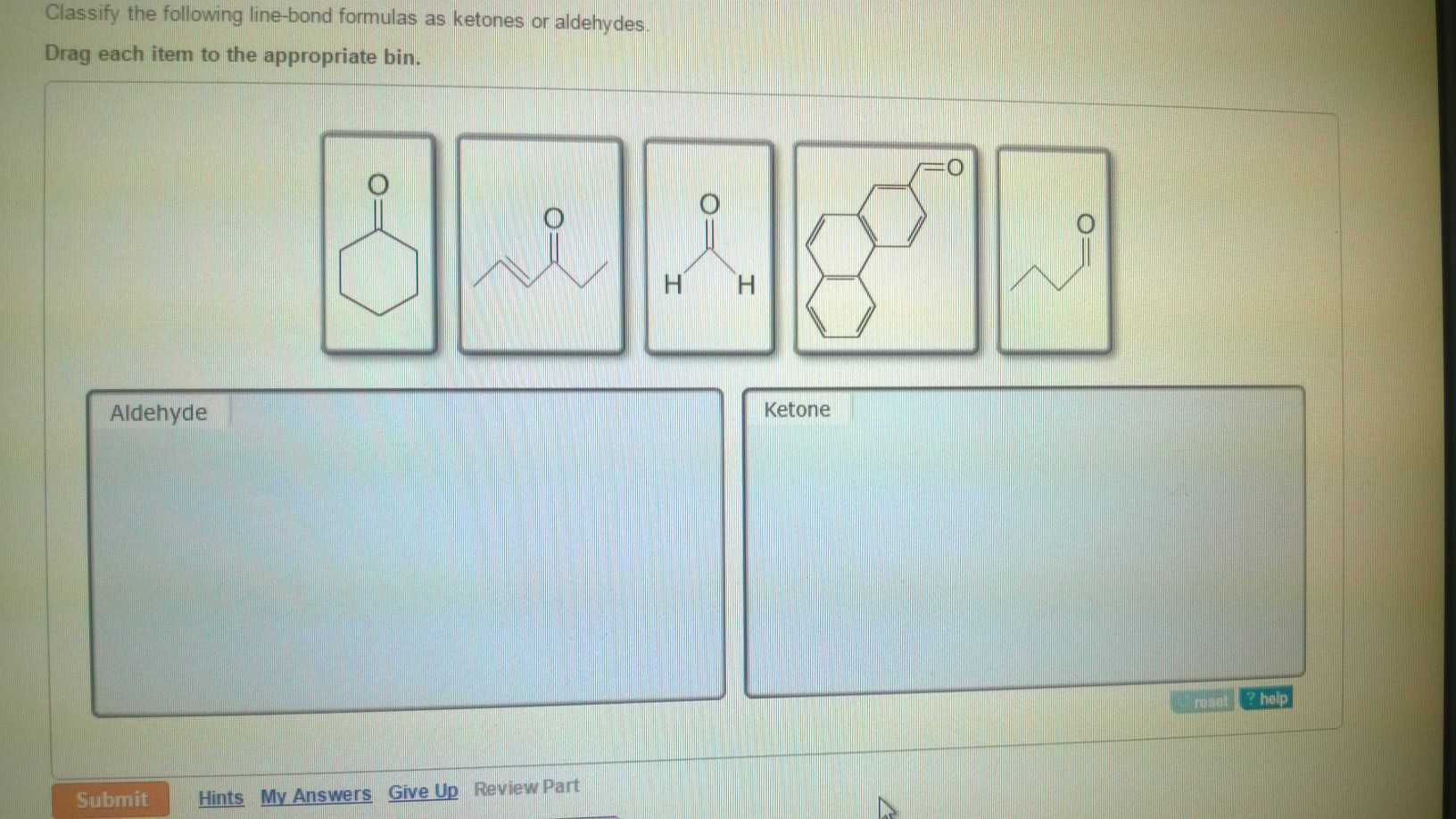
Part A Classify the following line-bond formulas as ketones or aldehydes.
. Drag the appropriate items to their respective bins. If neither of these substituents is hydrogen the compound is a Ketone. An aldehyde is another organic compound that has a functional group having the structure -COH.
Which of the following names does not fit a real compound. Drag each item to the appropriate bin. The carbonyl group a carbon-to-oxygen double bond is the defining feature of aldehydes and ketones.
Both compounds are simple organic compounds. The stem names of aldehydes and ketones are derived from those of the parent alkanes defined by the longest continuous chain LCC of carbon atoms that contains the functional group. For an aldehyde drop the -e from the alkane name and add the ending -al.
The molecule below contains both an aldehyde and a ketone functional group. Aldehyde- short structure with 2 carbons double bonded oxygen on 1st carbon. Classify the following line-bond formulas as ketones or aldehydes.
Water is a polar solvent. In an aldehyde and a ketone. Classify each compound as an aldehyde or a ketone.
The corners and ends of lines in a line-bond formula represent carbon atoms unless otherwise specified by a chemical symbol. Chapter 14 Homework There are two classes of carbonyl compounds. Methanal is the IUPAC name for.
Ketones are organic molecules that have the molecular structure RC OR. Here are some simple IUPAC rules for naming aldehydes and ketones. Science Chemistry QA Library Classify the following line-bond formulas as ketones or aldehydes.
Classify the following line-bond formulas as ketones or aldehydes. The dipole-dipole interactions are weak between propane and water. The molecule below contains both an aldehyde and a ketone functional group.
The carbon atom of this group has 2 remaining bonds that might be occupied by aryl or alkyl or substituents. 10421 442 PM HW CH 9 1317 Correct Part B Classify the following line-bond formulas as ketones or aldehydes. It can form hydrogen bond with water molecule.
View Available Hint s Soluble Not very soluble. Tap card to see definition. Aldehydes have at least one hydrogen atom attached to the carbonyl carbon which means that CO must appear at the end of a carbon chain.
Contains a carbon double bonded to an oxygen. It can form hydrogen bond with water molecule. Remember that carbon must have 4 bonds.
A ethanal B 3-methyl-2-pentanal. The stem names of aldehydes and ketones are derived from those of the parent alkanes defined by the longest continuous chain LCC of carbon atoms that contains the functional group. Up to 256 cash back -Aldehydes are more substituted versions of ketones.
Ester- Long structure with 2 carbons then an oxygen the 2 carbons doubled bonded oxygen on the 2nd carbon. Drag each item to the appropriate bin. Ketones do not have any hydrogen atoms attached to the carbonyl carbon because CO CO occurs between alkyl groups.
In aldehydes at least one. Water is a polar solvent. If at least one is hydrogen the compound is an Aldehyde.
Aldehydes and Ketones are often called as methanoyl or formyl group. Classify the following line-bond formulas as ketones or aldehydes. Click card to see definition.
The following general formulas in which R represents an alkyl group and Ar stands for an aryl group represent ketones. Ketones are widely used for industrial purposes. How to interpret a line-bond formula In a line-bond formula bonds are represented as lines.
Select only the carbonyl carbon atom of the. Select only the carbonyl carbon atom of the aldehyde functional group. -Ketones contain a carbonyl group between two carbons while the carbonyl group in an aldehyde must be attached to a hydrogen.
A monosaccharide is a n ___ if the carbonyl group is on the end of the carbon chain. Aldehydes have at least one hydrogen atom attached to the carbonyl carbon which means that CO CO must appear at the end of a carbon chain. View Available Hints Reset Help H H.
Methanal is the IUPAC name for. If a line-angle formula shows a carbon atom with only three bonds the missing fourth bond is understood to go to a hydrogen atom Drag each item to the appropriate bin. Identify the functional group s present in each molecule.
Give the common name for each ketone. -Aldehydes contain an oxygen atom bound directly to a hydrogen atom while the oxygen atoms in ketones are only bound to cabon atoms. Ketones do not have any hydrogen atoms attached to the carbonyl carbon because CO occurs between alkyl groups.
Glyceraldehyde is an example of a n ___ because it has three carbon atoms. Here are some simple IUPAC rules for naming aldehydes and ketones. View Available Hints Reset Help Soluble Not very soluble Fof10001 New Tab Review Consta d below.
View Available Hint s Reset Help he H H. Classify the following line-bond formulas as ketones or aldehydes. Properties of Aldehydes and Ketones Part B Classify the following aldehydes and ketones as soluble in water or not very soluble in water.
If a carbohydrate like xylulose has five carbon atoms and a carbonyl group on the second carbon it is called a n _____. Chemistry questions and answers. Drag each item to the appropri.
For an aldehyde drop the -e from the alkane name and add the ending -al. Drag the appropriate items to their respective bins. Part B Classify the following aldehydes and ketones as soluble in water or not very soluble in water.
Drag each item to the appropriate bin. Ketone- Long Structure with 5 Hydrogens and double bonded oxygen on the 3rd carbon. Drag each item to the appropriate bin.
Solved Part B Classify The Following Line Bond Formulas As Chegg Com

Solved Classily The Following Line Bond Formulas As Ketones Chegg Com
Solved Classify The Following Line Bond Formulas As Ketones Chegg Com
Solved Aldehyde Or Ketone Copy Classily The Following Chegg Com
0 Comments